FDA Approves First in Human Study Using the TxBDC ReStore Device
March 2, 2020
On Feb. 20, 2020 the Texas Biomedical Device Center (TxBDC) at The University of Texas at Dallas received FDA approval to proceed with an early feasibility study using the ReStore Vagus Nerve Stimulator to enhance recovery following a spinal cord injury. Technical specifications about the device were published in the Journal of Neuroscience Methods in May 2019.
Spinal cord injury occurs when there is damage to the spinal cord that blocks communication between the brain and the body. Many SCI patients experience long-term loss of motor and sensory function that can lead to permanent disability for which there are currently no effective therapies.

Dr. Robert Rennaker, professor in the School of Behavior and Brain Science at UTD and associate director of the TxBDC led the development of the ReStore Stimulator for delivering Targeted Plasticity Therapy (TPT). TPT is a technique developed by researchers at TxBDC. It pairs hand and arm movements with precisely timed vagus nerve stimulation (VNS). Stimulating the vagus nerve causes the release of chemicals in the brain that enhance recovery during rehabilitation. These tools allow TxBDC researchers to deliver VNS during hand and arm movements at just the right time. Our earlier clinical studies show that TPT can double the benefit of physical therapy in chronic stroke patients compared to traditional rehabilitation.
“The ReStore stimulator is critical for translating Targeted Plasticity Therapy to patients suffering from neurological injuries and disorders” said Dr. Robert Rennaker, inventor of the ReStore stimulator. “Without this device, we would not be able to perform the clinical trials necessary to help improve the quality of life for these individuals.”
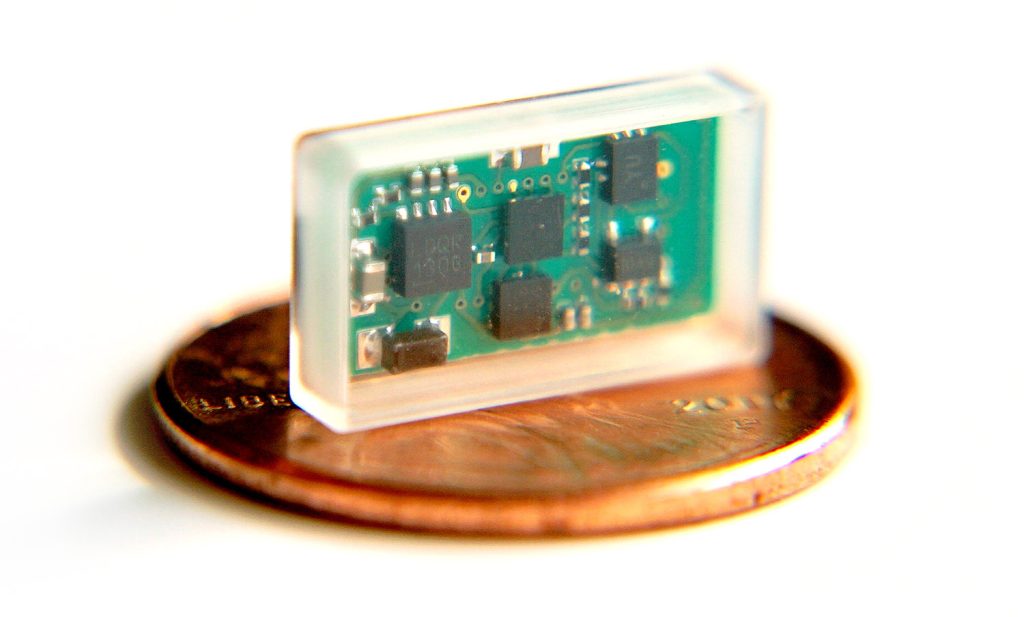
The ReStore system was designed to give patients the ability to continue therapy from the comfort of their own home using their cell phone or tablet. The ReStore device is a small, glass encapsulated, wirelessly powered implant that never needs to be replaced. The ReStore device is fifty times smaller than current VNS devices, which significantly reduces surgery time.
“Spinal cord injury affects more than 300,000 people in the US” said Dr. Jane Wigginton, Chief Medical Officer at TxBDC. “Patients need a real solution so they can get back to fully living their lives.”
“This is a momentous achievement for the TxBDC. Funding from W.W. Caruth, Jr. Fund at Communities Foundation of Texas, Texas Instruments, National Institutes of Health and DARPA made this possible” said Dr. Michael Kilgard. “I am so proud of the TxBDC team. The design, development, manufacturing and testing of an implantable system performed entirely at a university is unparalleled. This is a rare and unique accomplishment.”
Clinical trials using TPT to enhance recovery following a spinal cord injury are expected to begin in the Summer of 2020. Trials will be conducted in Dallas, TX with our clinical partners at Baylor Scott & White and UT Southwestern Medical Center. Learn more about clinical trials.

