FDA Approves a Second Clinical Trial Using the TxBDC ReStore Device to Improve the Treatment of PTSD
May 14, 2020
On April 15, 2020 the Texas Biomedical Device Center (TxBDC) at The University of Texas at Dallas received FDA approval to proceed with an early feasibility study using the ReStore Vagus Nerve Stimulator to enhance prolonged exposure therapy for treating Post Traumatic Stress Disorder (PTSD).
Post-traumatic stress disorder (PTSD) is a condition in which individuals feel anxiety and panic when reminded of a traumatic event. According to the National Center for PTSD, approximately 7 to 8 percent of the U.S. population will have PTSD at some point in their lives.
Researchers at TxBDC have developed a therapy to help PTSD patients reengage in life. This therapy uses stimulation of the vagus nerve during prolonged exposure therapy to rewire neural circuits, enhance learning, and improve recovery. We call this approach Targeted Plasticity Therapy (TPT). TPT uses vagus nerve stimulation during exposure therapy to reduce the fear response by helping the participant learn that they are safe.

How does it work?
Delivering this therapy depends upon an app called ReThink. The patient visits with their licensed therapist once a week. During the visit the therapist opens the ReThink app on their smartphone and records the therapy, triggering the ReStore device at specific times during therapy. The patient then takes the smartphone home to listen to their weekly recorded sessions. This therapy helps people learn that memories and reminders of their traumatic experience are safe. It reinforces that smells and objects that remind them of their traumatic experience are safe, which in turn reduces the fear and anxiety that occur with PTSD.
Office Therapy
The patient participates in therapy with a licensed therapist once a week.

The therapist uses a smartphone app to record the therapy and the triggers, which activate the vagus nerve stimulator.
Home Therapy
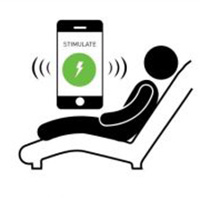
The patient uses a smartphone app to listen to their weekly recorded therapy sessions while receiving vagus nerve stimulation at home.
“The ReStore stimulator is critical for translating Targeted Plasticity Therapy to patients suffering from neurological injuries and disorders” said Dr. Robert Rennaker, inventor of the ReStore stimulator. “Without this device, we would not be able to perform the clinical trials necessary to help improve the quality of life for these individuals.”
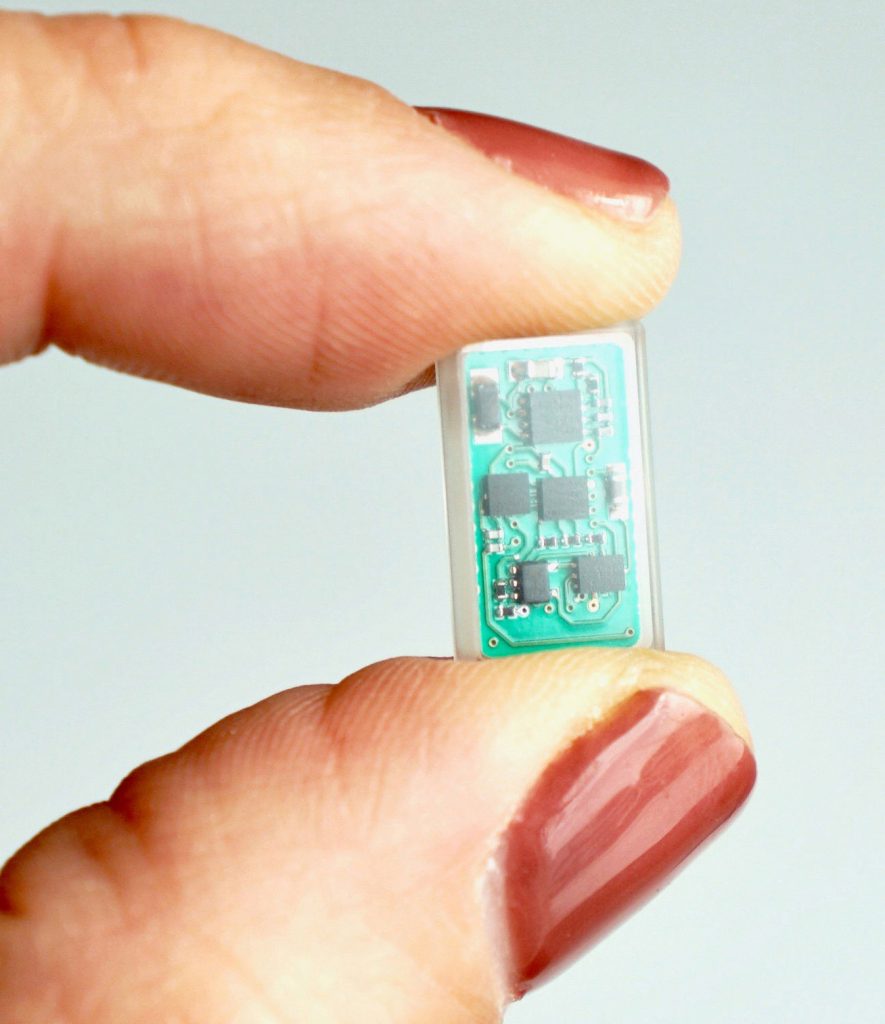
The ReStore system was designed to give patients the ability to continue therapy from the comfort of their own home using their cell phone or tablet. The device itself is a small, glass encapsulated, wirelessly powered implant that never needs to be replaced. The ReStore device is fifty times smaller than current vagus nerve stimulators, which significantly reduces surgery time. Technical specifications about the device were published in the Journal of Neuroscience Methods in May 2019.
“This is a momentous achievement for the TxBDC” said Dr. Michael Kilgard. “I am so proud of the TxBDC team. The design, development, manufacturing and testing of an implantable system performed entirely at a university is unparalleled. This is a rare and unique accomplishment”
Clinical trials using TPT to enhance prolonged exposure therapy for treating PTSD are expected to begin in fall 2020. Trials will be conducted in Dallas, with our clinical partners at Baylor Scott & White and UT Southwestern Medical Center. Learn more about clinical trials.
Read more about the Texas Biomedical Device Center.
