A Promising Treatment for Stroke
September 8, 2021
Researchers at The University of Texas at Dallas (UTD) along with physicians at sites throughout Europe and the United States, have together gained FDA approval last week for a new therapy for patients recovering from stroke. The therapy involves stimulating a specific nerve called the vagus nerve during physical therapy and has been found to improve/increase the strength and agility patients can recover compared to those who do not undergo the same therapy. Results of this work were published in the Nov. 2018 issue of Stroke.
“Moving the idea from concept to invention to therapy required the last decade and the work of several hundred UTD students and scientists and the generosity of the Retirees of Texas Instruments who provided much of the funding to support this work,” says Michael P. Kilgard, Ph.D., a professor of neuroscience at UTD and the leader of the research effort. “We are really excited to see our research led to a treatment that can improve patient outcomes.”
The lion’s share of the early research was done by The Texas Biomedical Device Center (TxBDC) at The University of Texas at Dallas, which focuses on developing engineering solutions to medical conditions with no treatment options and was founded in 2012 by Kilgard and Rob Rennaker, Ph.D., a professor of neuroscience and the Texas Instruments Distinguished Chair in Bioengineering at UTD.
TxBDC researchers focused on stroke because the cause is well understood. Neurons die when a blood clot restricts blood flow to the brain. Every year loss of these neurons leaves millions of people unable to move on one side and struggling to perform even basic activities. Physical therapy can help survivors recover some of the lost function, but modern medicine has no therapies after the damage is done. “Stroke rehabilitation has changed little in a hundred years and TxBDC researchers thought there must be a better way to recover from a stroke.” Need to attribute this to a person, preferably one of your MD collaborators.

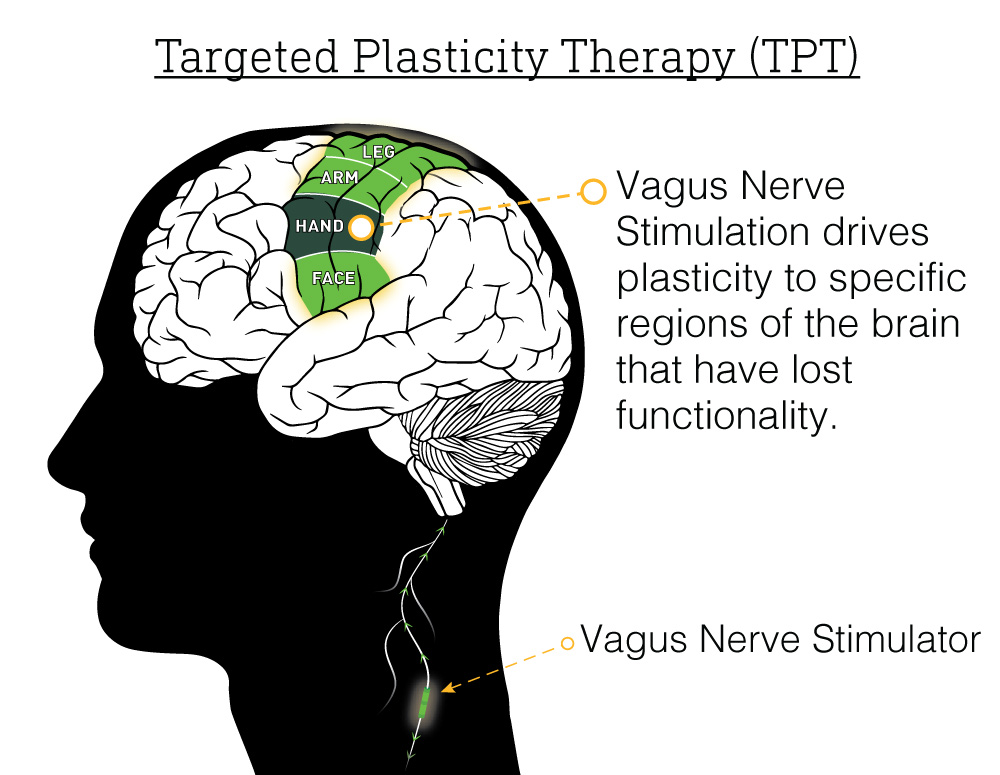
The potential for the brain to change, called brain plasticity, has long been proposed as a method to improve recovery from brain injury. The challenge has always been figuring out how to control brain plasticity to aid recovery. For his PhD research project, then-UTD neuroscience graduate student Ben Porter, PhD observed that electrically stimulating certain nerves in the neck could coax the brain into growing more nerve cells. Expanding his work from studying plasticity in the part of the brain responsible for processing sound, Porter found in rodents/rats that stimulating the vagus nerve while the rat was moving resulted in an increase in nerve cells in the part of the brain responsible for that specific movement. (link).
The first direct evidence that VNS could be used to enhance recovery from stroke was collected by Navid Khodaparast, Ph. D and Seth Hays, Ph.D. in 2013. The team produced strokes in rats and showed that VNS greatly improves the effectiveness of physical therapy in improving strength and agility (link). Their pioneering studies confirmed that VNS improved recovery whether the rats were young or old (link) and whether the injury occurred recently or long ago (link).
Simply showing that VNS helps rats get better does not prove that VNS can help people. To move the therapy to people, Eric Meyers set his sights on understanding precisely how VNS works. Eric’s dissertation revealed that VNS triggers the release of one of the key neurotransmitters that regulates brain plasticity, called acetylcholine (link). Eric also showed that pairing VNS with one task could improve performance of other tasks. Eric even figured out a way to track the new neural connections occurring on both sides of the brain which help to restore lost function.
David Pruitt evaluated precisely how much VNS was needed to enhance stroke recovery and found that VNS only helps recovery at an intermediate dose and not at low or high doses (link).
Collectively, these animal studies made a strong case that VNS should be tested in people recovering from stroke. Navzer Engineer, a UT Dallas graduate (PhD’04) helped translate the work of the TxBDC researchers into several clinical trials in his role as Chief Scientific Officer for MicroTransponder, whose engineers developed the Vivistim® Paired VNS™ System to enable the first human trial of this new technology. UTD researchers collaborated with MicroTransponder and a Scottish stroke neurologist named Jesse Dawson to test the therapy. The first study confirmed that VNS could double or triple the benefit of physical therapy in people with chronic stroke (link). Mike Kilgard and Jesse Dawson later reported the first clinical evidence that VNS could also improve sensory function lost to stroke (link).
Teresa Kimberley at Massachusetts General Hospital led researchers across different four hospitals and provided the first trial of VNS after stroke using a study design that was double-blinded and placebo-controlled (link), which was published in 2018. This gold-standard clinical evidence confirmed the benefit of VNS and convinced the FDA to approve a massive clinical trial that would serve as the final test of VNS as a stroke therapy. The trial was conducted at nineteen stroke rehabilitation centers across the world from Scotland to Texas. The results of the trial, published in April of 2021, provided further evidence that VNS during rehabilitation improves recovery from stroke compared to rehabilitation without VNS (link). The trial also proved that VNS was safe.
The Vivistim® Paired VNS™ System is the first FDA-approved neurotechnology proven to treat stroke related impairment. Stroke patients will soon be able to consult with their physicians to determine if VNS therapy is appropriate to aid their recovery of hand function after ischemic stroke.
The approval of VNS for stroke and FDA designation as a “breakthough” (link) opens the door to testing VNS as a treatment for other brain disorders. TxBDC researchers at UTD will continue to coordinate its large team of scientists, engineers, physical therapists, and doctors to develop new therapies for other untreatable conditions.
Biomedical research is inherently risky. TxBDC is proud of its mission to innovate, to de-risk new technology through preclinical and clinical studies, and ultimately to transition new therapies to industry partners for commercialization.
VIDEO: Stroke Research – Brief Overview
GRAPH: Rehabilitation vs. VNS + Rehabilitation
