UTSW, UT Austin, and UT Dallas Collaborate for MS Safety Trial
May 11, 2017
The Texas Biomedical Device Center at The University of Texas at Dallas will collaborate with UT Southwestern and The University of Texas at Austin for a 5 patient safety trial using Targeted Plasticity Therapy (TPT) to accelerate recovery of arm function in individuals with motor deficits resulting from Multiple Sclerosis (MS). MS is a disease that attacks the central nervous system causing symptoms such as numbness in the limbs, muscle weakness, poor coordination, and even paralysis. Although some patients fully recover, for many patients, arm and hand movement deficits persist.
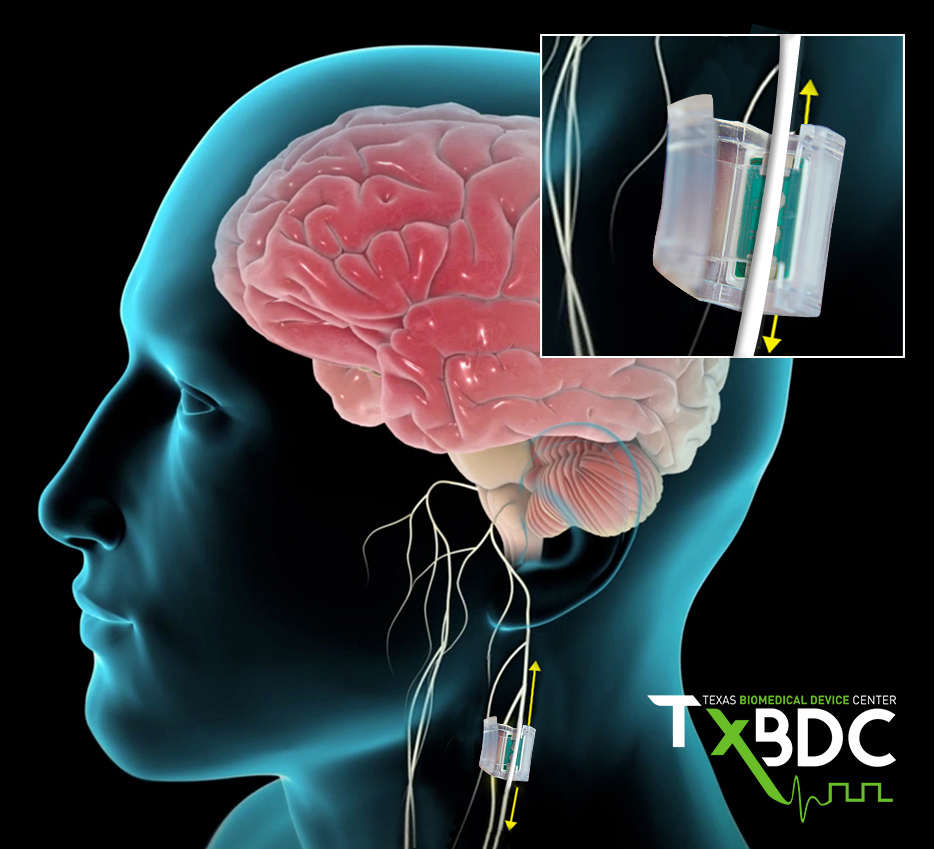
This trial will use vagus nerve stimulation (VNS) to release chemicals in the brain which are known to enhance recovery. Pairing VNS with specific movements during rehabilitation may therefore improve recovery of motor functions.
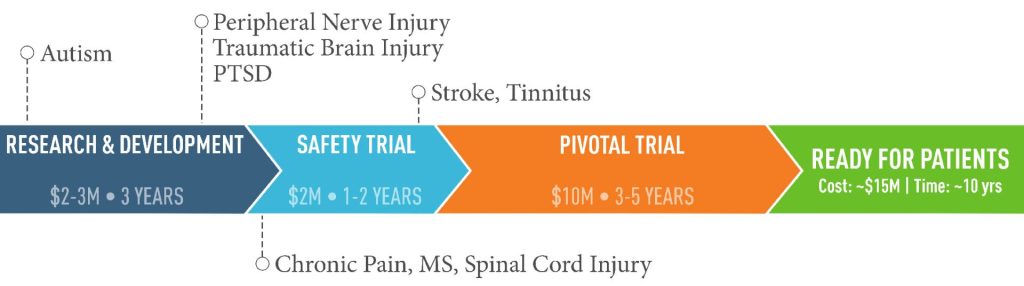
Results from pre-clinical research conducted at the Texas Biomedical Device Center demonstrate that TPT enhances recovery of motor function in peripheral nerve injuries, spinal cord injuries, and brain injuries. This same approach has been shown to be safe and demonstrates great promise in stroke patients with arm and hand deficits. These findings provide strong evidence that MS patients will benefit from TPT as well. Once Upon a Time Foundation provided $1M of the $2M needed to complete the first step in getting this therapy to those in need; the safety trial. The graph below illustrates current progress and future timeline towards delivering TPT to patients.
“For more than 30 years the focus has been on making a difference for those who suffer from multiple sclerosis,” said Dr. Frohman. “Dr. Rennaker and I both believe it’s imperative to develop better therapeutic care. I am grateful for this gift and what it could mean for MS patients.”


Right: Dr. Robert L. Rennaker
This clinical trial will be led by Managing Director of the Multiple Sclerosis and Neuroimmunology Center at UT Austin, Teresa Frohman, and Director of the Multiple Sclerosis and Neuroimmunology Center Dr. Elliot Frohman. All surgeries will be led by nationally recognized otolaryngologist, Dr. Bradly Marple and his colleges at UT Southwestern. Dr. Robert Rennaker, Executive Director of the Texas Biomedical Device Center at UT Dallas will provide TPT experience while leading regulatory and engineering efforts.
